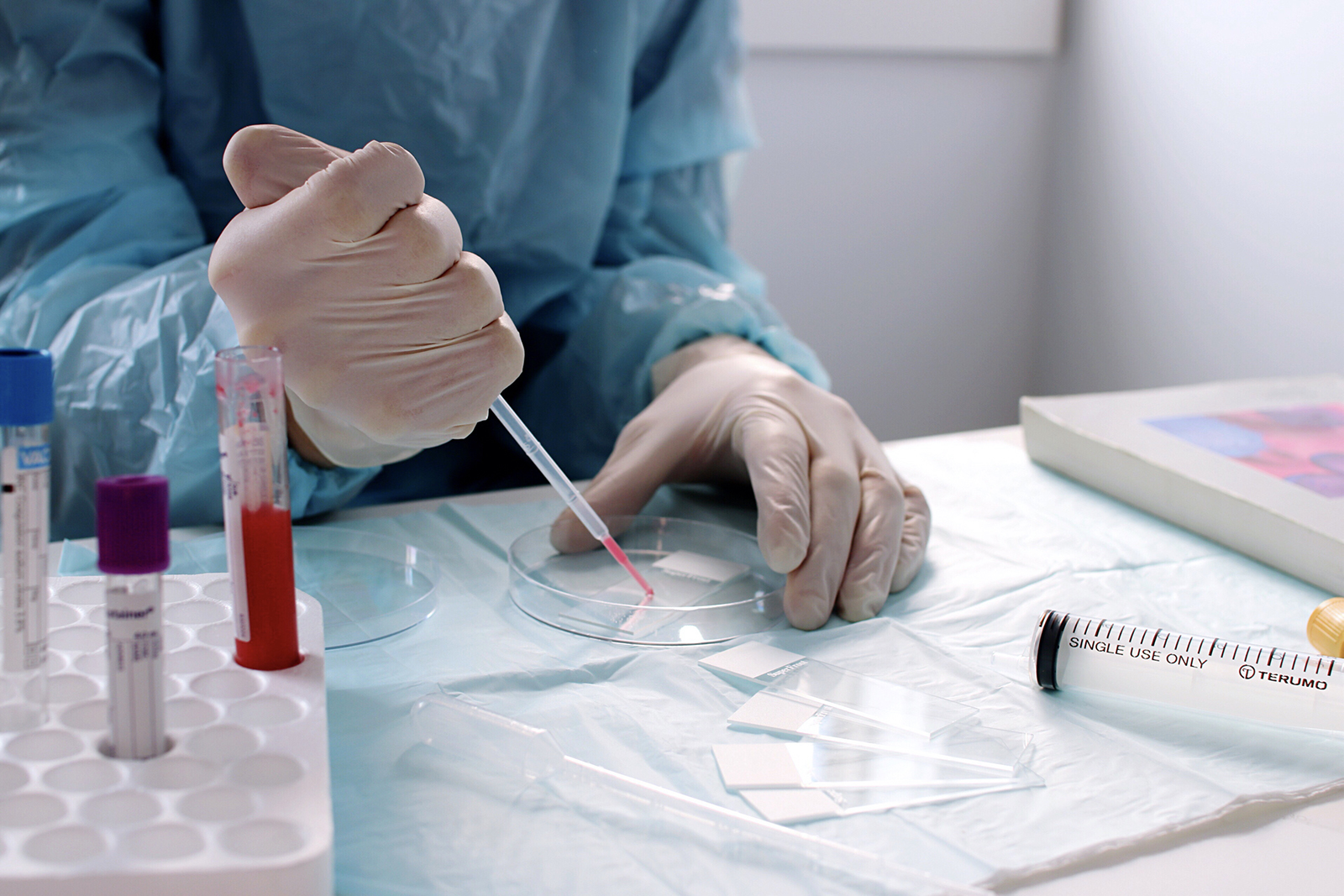
Mel-Mont Medical specializes in designing, developing, and marketing state-of-the-art technologies and medical services that preserve life through the prevention and early diagnosis of RNA-virus-related pathologies. Our experienced team and top-end equipment ensure the safety and confidence of our patients.
PATENTED DEVICES AND MOLECULAR TECHNOLOGIESOur Vision
To be leaders in molecular testing by managing a diversified portfolio of proprietary technologies and medical devices for secondary prevention and early identification of DNA and RNA virus-related pathologies (HPV, respiratory viruses).