Having high-risk Human Papillomavirus is NOT THE SAME as having Cervical Cancer.
Most HPV infections are asymptomatic and short-lasting and are cleared by the body’s own immune system. However, infections caused by one or more of the 14 high-risk HPV genotypes (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68) can be long-lasting and lead to precancerous lesions, which can develop into Cervical Cancer if left untreated.
Cervical Cancer is the only cancer that is 100% preventable.
World figures: 2
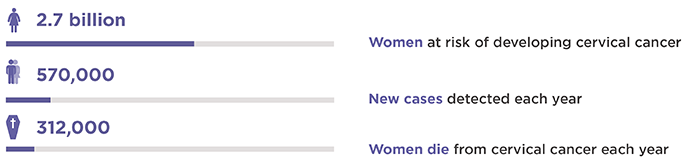
2 World Health Organization. International Agency for Research on Cancer IARC. December 31, 2018